Covid-19 Updates
What Does it Mean to be Fully Vaccinated?
The Centers for Disease Control and Prevention (CDC) is striving to shift the language on what it means to be “fully vaccinated” without changing it officially. Their mission is to encourage booster shots to those who have already received their primary COVID-19 vaccination series, while inviting other Americans to begin the series. This has led the CDC to practice caution when officially shifting what it means to be “fully vaccinated.”
This communication to the public is ever evolving and it must be altered with caution. Because transforming the definition could cause a chain of events that may create confusion and disruption in many cities around the US with vaccine mandates. Although some areas have their own requirements, such as requiring a booster, most base their requirements on what the CDC recommends.
For those reasons, the CDC is slowly moving away from using “fully vaccinated” language. The new phrase is asking everyone to be “up to date” on their shots. The director of the CDC stated that the term “up to date” is consistent with how public health has historically viewed recommended vaccines.
Sources: Tina Reed. “The Shifting Definition of Fully Vaccinated,” Axios, 2022.Alison Durkee. “CDC Not Changing “Fully Vaccinated” Definition to Include Booster — But People Won’t Be “Up to Date” On Shots Without One,” Forbes, 2022.
Why are Covid-19 Treatments so Hard to Get?
The federal and state governments are currently in control of distributing the limited antiviral COVID-19 treatments available. These antivirals are vital as they can reduce the risk of hospitalization and death by up to 89%.
The path from diagnosis to treatment is within a short period and can be challenging. The biggest obstacles to overcome are obtaining access to testing, finding a provider who can prescribe and finding the medication within your area.
Currently, the most at-risk patients should have a plan of action to access these medications if and/or when they test positive. When more pills are available, steps must be in place to streamline this process.
Source: Tina Reed. “1 Big Thing: Why COVID Treatments Are So Hard to Get,” Axios, 2022.What Delayed Routine Health Care During Pandemic?
It is of no surprise that the pandemic is taking a toll on physical and mental health within the US. A new study shows that more than 4 in 10 workers have delayed routine health care appointments.
According to the survey within the study, the top five reasons for delaying care are:
- Fear of contracting COVID-19 (47%)
- Difficulty getting an appointment (29%)
- The need to cancel appointments due to COVID-19 restrictions/requirements (25%)
- Fear of other illnesses (24%)
- Not a current priority (21%)
Dr. Adam Seidner, the Hartford’s chief medical officer, recommends the following in order to better engage with workers and promote overall wellness:
- Offer benefits and resources that address the overall well-being of their workforce — encompassing physical health, mental health, as well as financial resilience
- Communicate more often to employees to remind them of the benefits and services that are available
- Lead by example by making your own appointments a priority
- Offer the flexibility employees need to make their appointments a priority
How Long Do COVID-19 Vaccines Last?
How long the COVID-19 vaccine remains effective is based on the strain you have been exposed to, how many shots you have received and what strain you are trying to prevent. The effectiveness of vaccines decreases over time, especially as new strains come along. According to a recent study, efficacy against infection decreased by 20 – 30% six months after individuals received their third shot.
As of now, the US FDA and CDC recommend a second booster shot for those 50 and older and those who are immunocompromised. Preliminary studies found that while a fourth shot did not do much to prevent infection further, it did decrease the odds of hospitalization and death among the elderly.
Sources: Clifford Colby, Dan Avery. “These Are the People Who Should Get a Second COVID Booster Shot,” CNET, 2022.Clifford Colby, Dan Avery. “Here’s How Long Your COVID Vaccine Booster Provides Protection,” MSN, 2022.
Rising Costs in Healthcare
Launch of Mark Cuban Pharmacy
January marked the opening of Mark Cuban Cost Plus Drugs Company (MCCPDC). Dr. Alex Oshmyansky is the CEO and brought the idea to Cuban with the overarching goal of total transparency with pricing.
There are currently over one hundred medications being sold for various disease states, and the pricing model charges a 15% margin on the manufacturing price and a $3 pharmacy labor fee. The list of medications, pricing breakdown and fees can all be found at costplusdrugs.com.
However, it’s important to note that these prescriptions are not processed through insurance, and customers will be paying fully out of pocket. Also, MCCPDC will not transfer prescriptions from other pharmacies, therefore customers will need to request new prescription orders from their prescribers.
Even with insurance not being accepted, the discounts are often high enough to still be a better value. This appears to be the first step in the company’s plan to cause disruption in the pharmaceutical space, as there are plans to manufacture its own products as well.
Sources: Jamie Reno. “Mark Cuban’s New Online Pharmacy: How It Could Disrupt the Prescription Drug Industry,” Healthline, 2022.Lisa Kim. “Billionaire Mark Cuban Opens Online Pharmacy to Provide Affordable Generic Drugs,” Forbes, 2022.
6.6% Average Increase in Drug Prices
With a heavy political focus on medication costs, drugmakers appear to have made moderate increases in drug prices. This is especially apparent considering the 7% overall consumer inflation rate. Price increases are typically common at the beginning of a new year. This increase is noticeable with various medications, including some treatments for cancer, diabetes and other disease states.
The previous year saw much higher increases in drug pricing which led to high scrutiny from congress. Many drugmakers say they do not see benefits from the price increases either because discounts and rebates are being provided to insurers and pharmacy-benefit managers.
According to an analysis from Rx Savings Solutions, a software company that helps find the lowest cost alternative medications, there will be 866 products from 150 manufacturers raising prices. Some of the price increases are on brand medications, and there are also some generic medications having price increases as well.
Source: Joseph Walker. “Drugmakers Raised Prices by 6.6% on Average Early This Year,” The Wall Street Journal, 2022.
Prescription Medications Price Increases 2.5% Since Beginning of Pandemic
Data from GoodRx shows a 2.5% increase in prescription medication costs since the start of the COVID-19 pandemic. Several other commodities and services have increased in price at a higher rate than prescriptions, including car rentals, tobacco, beef and moving expenses. Although historically, this has not been the case as drug prices usually increase faster than the inflation rate.
“According to an analysis from Rx Savings Solutions, a software company that helps find the lowest cost alternative medications, there will be 866 products from 150 manufacturers raising prices.”
Earnings have not been able to keep up with increasing drug costs. Since 2014 hourly earnings have gone up by 28%, while prescription medications have increased by 35% over the same period. While unemployment rates are moving in the right direction, there is clearly still a gap. Alarmingly, 1/3 of Americans have reported not taking a prescription medication due to cost.
Source: Jeff Lagasse. “Prescription Drug Prices Have Risen 2.5% Since the Start of the Pandemic,” Healthcare Finance, 2022.Credit Lines Offer Help for Medical Costs
Companies like CareCredit are providing consumers lines of credit to help cover out-of-pocket medical expenses. This can be highly beneficial for unexpected medical events. For instance, people are catching up on accessing care, which was put off during the pandemic, and credit lines can even be used for pet needs.
A PYMNTS report showed patients are paying about $810 for healthcare visits. So although the backlog of patients seeking routine care starts to ease, there will always be unexpected costs and deductibles where a line of credit can be impactful. Tools like these can help consumers manage medical expenses and also aim to help health systems improve their debt collection.
Source: Alan Goforth. “Health-Specific Credit Lines Aim to Address Out-Of-Pocket Expenses,” BenefitsPRO, 2022.Hospitals Charging Double for Same Medications Compared to Pharmacies
A recent study from AHIP has shown a large disparity in medication costs depending on where they are being filled. The study looked at ten medications administered by providers from 2018 to 2020. It showed that the cost in hospitals was on average $7000 higher than in specialty pharmacies.
The study also looked at medications given through the physicians’ offices. Those medications were on average $1400 more than in the pharmacy setting. Focusing on where the medications are administered is an opportunity for healthcare savings which often gets overlooked, and studies like this add more light to this topic.
Source: Jeff Lagasse. “Hospitals Charge Double for Specialty Drugs Compared to Pharmacies, AHIP Findsa>,” Healthcare Finance, 2022.Biosimilars Boom
Biosimilars Are Projected to Reduce Drug Costs
8 in 10 Americans say prescription drug costs are unreasonably high. “Biosimilars have the potential to lower spending on biologic drugs that account for a rapidly increasing share of overall United States prescription drug spending,” Andrew Mulcahy, a senior policy researcher at RAND, said in a statement.
Biologics are complex drugs manufactured in living systems that include insulin, monoclonal antibodies to block inflammation in rheumatoid arthritis, and a range of drugs to treat cancer, multiple sclerosis, and other serious diseases.
Biosimilars are FDA-approved, lower-cost biologic medicines with the same safety and efficacy as their reference counterparts. Biosimilars are used to treat many chronic, life-threatening conditions, including cancer, diabetes, Crohn’s disease, arthritis, macular degeneration, and more. The approval pathway for biosimilars was established by the 2010 Biologics Price Competition and Innovation Act (BPCIA), within the Affordable Care Act (ACA), leading to the first biosimilar Zarxio being approved in 2015 in the United States.
According to AJMC, biosimilars are projected to reduce drug costs by $133 billion in the United States by 2025. In addition to being a lower-cost treatment to brand biologics, biosimilars also lower the cost of other biologics. In fact, a 2021 study found the average price of brand biologics was estimated to be 56% higher without biosimilar competition — and up to 150% higher for some cases.
About two-thirds of savings from biosimilars were from those expected to be launched between 2021 and 2025. Investigators found that adalimumab, Humira’s biosimilar from AbbVie, was the greatest contributor at about $19.5 billion. The remaining one-third of savings was estimated from greater use and lower prices for already-marketed biosimilars.
Source: Ashley Gallagher. “Study: Biosimilar Drugs Could Generate $38.4 Billion in Savings Over 5 Years,” Pharmacy Times, 2022.Humira Biosimilar: The Upcoming Release
AbbVie Inc. made its arthritis treatment Humira the best-selling medication in the world. Now, after years of wrangling, rivals are finally set to bring a wave of lower-cost versions to the US.
The arrival of the Humira copycats will be a pivotal test for a class of treatments that advocates have long said could help fence in runaway drug costs. Biosimilars – cheaper versions of complicated treatments made from living cells –remain a relatively small market in the US. Part of this reason is that makers of big-name biologic drugs have fought hard to protect themselves from the competition.

Humira is the biggest and best-protected of them all. Since AbbVie’s parent Abbott Laboratories introduced the drug in 2003, it’s brought in almost $193 billion in sales through the end of 2021. Partly because of an imposing wall of patents. AbbVie fended off prospective rivals for years, but now settlements reached with companies who want to make Humira clones for the US, where it sold $17.3 billion last year, are set to kick in.
“According to AJMC, biosimilars are projected to reduce drug costs by $133 billion in the United States by 2025.”
But there’s a key difference with Humira that may give generic-drug makers an advantage. Unlike physician-administered drugs, Humira is dispensed by pharmacies, giving the pharmacy benefit managers that purchase products for companies and insurers greater discretion in choosing whether and when to use substitutes.
“As more biosimilars come on in the market, payers are incentivized to increase adoption,” Bloomberg Intelligence analyst Duane Wright said. “But it’s not going happen right away.”
In general, biosimilars have fared better in most European countries than in the US because of lower legal barriers and the power of government health systems. Let’s observe Amgen’s Amjevita, which launched in Europe in 2018 when it sold $11 million in its first three months. It’s now the continent’s leading biosimilar, with $439 million in 2021 sales.
Source: Fraiser Kansteiner. “Organon CEO Pins High Hopes on Upcoming Humira Biosimilar Launch,” FiercePharma, 2022.Revlimid: The Growing Demand
According to a new report published by Polaris Market Research, the global blood cancer drugs market is anticipated to reach USD $55.6 billion by 2025. The demand for blood cancer drugs is primarily driven by growing death incidences by blood cancer, and continuous innovation for developing novel treatments with the help of several ongoing clinical trials. Moreover, increasing research and development of biological and targeted therapies as treatment will spur the blood cancer drugs market during the upcoming period.
Since Bristol Myers Squibb acquired Celgene and its mega blockbuster Revlimid, it has girded for the day the multiple myeloma superstar would face generics. In many European countries, that day is here.
Novartis’ Sandoz revealed that it has launched its generic version of the medicine in 19 countries in Europe. According to reporters, German generics maker Stada Arzneimittel also has launched its Revlimid generic in Europe. Last September, Sandoz launched its generic lenalidomide in Canada. Meanwhile, the first Revlimid generics are scheduled to reach the huge United States market next month.
The company recently reported that the drug generated $12.8 billion for BMS last year. With the flood of incoming competition, BMS expects sales of Revlimid to drop to between $9.5 billion and $10 billion this year.
Last September, Sandoz launched its generic lenalidomide in Canada. The generic Revlimid was launched in the US in March 2022, although with limited availability, likely until 2026.
Sources: Kevin Dunleavy. “Sandoz Launches Generic Revlimid in 19 Countries in Europe, Opening a Flood of Competition for BMS’ Megablockbuster,” FiercePharma, 2022.Drug Updates
FDA Approves First Generic of Restasis
In early February, the US Food and Drug Administration (FDA) approved the first ever generic for Restasis eye drops. Restasis is a cyclosporine eye drop product, packaged in single-use vials and used to increase natural tear production for people with chronic dry eye conditions.
It’s unknown exactly how cyclosporine treats chronic dry eye. However, it’s a type of immunosuppressant medication, so it’s thought that it works to stop immune cells in your eyes from creating inflammation/swelling. The inflammation causes symptoms like scratchy eyes for people with chronic dry eye conditions, so relieving this inflammation is thought to allow your eyes to make more natural tears.
Restasis was first approved nearly 20 years ago, and there has not been a generic version available until now. Some generic medications are more complicated to produce due to active ingredient formulations or a specific route of delivery that may present certain challenges. With these challenges in production, it is not uncommon for complex drugs, like cyclosporine eye drops, to see significant delays in generic product approvals or without a generic version altogether.
It’s important to note that generic medications are subjected to thorough testing to prove that they work the same way as the original brand version. The FDA holds generic manufacturers to the same high standards as brand medication manufacturers. So, when a generic medication is FDA approved, this means it’s been verified to be as safe and effective as the brand medication. Therefore, when there is a generic available, pharmacists can automatically substitute the generic medication.
Source: US FDA. ““FDA Approves First Generic of Restasis,” GovDelivery Bulletin, 2022.Scientists May Have Cured HIV in a Woman for the First Time
The woman identified only as the “New York patient” may be the first woman cured of HIV by a stem cell transplant method. There had already been three male patients reported to be cured of HIV via the method, as well as two other female patients whose own immune systems have seemingly eliminated the virus naturally.
The first reported case of success was published in 2008 after a patient with acute myeloid leukemia (AML) received a stem cell transplant from a donor who apparently had a rare genetic anomaly that allows immune cells, normally attacked by HIV, to resist infection.
When treating cancer, the stem cell transplant is intended to replace the patient’s immune system with that of the healthy donor. The patient’s immune system must first be wiped out using chemotherapy, with or without radiation. The healthy donor stem cells will then take over, making new, healthy immune cells after transplantation.

It is critical to note that this process is not considered ethical to treat only HIV due to the toxic nature of chemotherapy. All patients treated with this method have had cancer or some other condition, making them a candidate for stem cell transplant, in addition to their HIV status.
Researchers estimate that there may only be about 50 people per year in the US that could benefit from this procedure. One of the greatest challenges is finding donors with the necessary genetic anomaly for HIV resistance. The genetic factor has been linked largely to people with northern European ancestry, but even then, the rate is estimated at only 1%.
So far, the “New York patient” has been in remission from her leukemia for four years, and she was able to stop HIV treatment three years after her stem cell transplant. It has been at least 14 months since then, and she still has not experienced any relapse in HIV infection.
Source: Benjamin Ryan. ““Scientists Have Possibly Cured HIV in a Woman for the First Time,” NBC News, 2022.Possible Label Expansion for Tremfya
The autoimmune biologic drug classes are seeing significant growth year over year as additional drugs are launched. These drugs are also found to treat additional conditions. Many autoimmune biologic drugs are initially launched for one indication and then later pursue drug trials to gather sufficient data to expand their indications or conditions they can treat.
Tremfya is a biologic drug that is currently used to treat the autoimmune skin conditions psoriasis and psoriatic arthritis. In 2021 there were about $2 billion in global sales attributed to Tremfya, nearly 60% greater than its sales in 2020.
Tremfya is currently in trial for the potential expansion to be used for both Crohn’s disease and ulcerative colitis. It is estimated that there are approximately 1 million Americans with ulcerative colitis and approximately 3 million Americans impacted by Crohn’s disease, so if approved, Tremfya could again see significant growth with expansion into these areas.
Source: Fraiser Kansteiner. ““J&J Lands Tremfya One-Two Punch in IBD with First Ulcerative Colitis Win and Long-Term Crohn’s Data,” FiercePharma, 2022.CDC Proposes New Opioid Guidelines
The CDC is considering updating its guidelines for prescribing opioid pain medications from specific dosage or duration limits to a more flexible approach. There is some concern that current guidelines may be misinterpreted or present barriers to medication for those in need. The current guidelines were released in 2016 as healthcare sought to get a handle on the opioid epidemic and reduce the risk of overuse or addiction.
Some groups, like the American Medical Association, feel the current 2016 guidelines are too rigid and have urged the CDC to remove the specific prescribing limits. On the other hand, the group Physicians for Responsible Opioid Prescribing, voiced concern that drug companies were driving the opposition as they may view the current restrictions as impactful to their sales.
The Washington Post reported stats from the CDC that “collective action has dramatically reduced opioid prescriptions from a peak of 255 million in 2012 to 143 million in 2020. But deaths from drug overdoses have continued to skyrocket. According to the CDC, nearly 92,000 people died of drug overdoses in 2020, with fentanyl by far the largest cause. Additionally, the data that supported the 2016 guidelines found that three days or less of pain medication would generally be sufficient; more than seven days of medication would rarely be necessary.
The CDC will decide whether to make the changes after the public comment period has ended, likely mid-2022.

The proposed guidelines encourage the use of non-opioid pain medications as first-line as well as non-pharmacological measures like physical therapy, massage and acupuncture. The new guidelines would not apply to hospice pain care or pain management for chronic, painful conditions such as cancer and sickle cell anemia. The CDC will decide whether to make the changes in mid-April 2022 after the public comment period has ended.
Source: Lenny Bernstein. ““CDC Proposes New Prescription Opioid Guidelines for Caregivers,” The Washington Post, 2022.Non-Opioid Pain Relief After Orthopedic Surgery
Several recent studies support alternative pain management methods as effective following some orthopedic surgeries. Patients included in the studies all received a nerve blocker before their procedure and then non-opioid pain medications such as acetaminophen, ibuprofen/naproxen, muscle relaxers and in some cases also a nerve pain specific medication, like gabapentin. The study results suggest that this regimen provided comparable, if not greater, pain management following these surgeries than opioid medications.
The risk of overuse leading to addiction is itself important enough to void opioid medications, when possible, but this non-opioid regimen would also help patients avoid other side effects too. Opioid pain medication also can cause significant drowsiness and dizziness that could lead to falls, especially after an orthopedic surgery – such as knee or hip procedures. Opioid medications are known to cause GI side effects frequently as well, and many patients need supplemental treatment for constipation while taking them.
Source: US News Staff. ““A Non-Opioid Way to Pain Relief After Knee, Shoulder Surgeries,” US News, 2022.Digital Tools
Smartwatches and Our Health
Wearable health and fitness devices are rapidly evolving from low-tech step counters to more advanced gadgets in the world of personal health. In fact, 30% of Americans use a wearable health device. The market has even expanded from watches and wristbands to skin patches, eyeglasses and clothing. They can be used to track, monitor and transmit data on heart rate and rhythm, blood pressure, body temperature, blood sugar levels, quality of sleep and even early warning signs of COVID-19 infection.
Fitbit started the trend about a decade ago, and since then, the wearables market has attracted other Big Tech players, including Apple, Google and Amazon. According to Fortune Business Insights, the market reached more than $36 billion in 2020 and is projected to top $114 billion by 2028.

There is increasing interest in incorporating the medical data from these wearables into the digital health system. Ideally, vital data would be continuously downloaded to primary care providers to track patients in real time, monitor their overall health and respond to any emergencies. However, physicians largely remain unconvinced the data is reliable and that patients’ health information is protected. If these factors can be addressed, physicians will be able to take better care of their patients.
Source: Bob Woods. ““What Devices like Apple, Google Smartwatches Are Beginning to Display About Our Health,” CNBC, 2021.Telehealth Expands Beyond Pandemic
The use of telehealth has declined since its peak in the pandemic. However, telehealth is still a viable option for patients, physicians and insurers. More people are utilizing in-person care in the most recent months of the pandemic. In fact, older adults have shown lower retention of telehealth. Younger patients have continued to use telehealth at higher rates than the elderly. We have also seen that rural and urban patients use telehealth at similar rates. The same goes for men and women telehealth users. Last, telehealth used for chronic condition management has continued to decline but is still being used more than in pre-pandemic times.
In the future, telehealth is expected to become sustainable beyond the pandemic. The US Department of Health and Human Services is giving $55 million to improve access to community health centers. These health centers provide better access to telehealth by expanding digital disease monitoring and enhancing health information technology. There are also a few bills under consideration at the federal level that would also remove the barriers to telehealth. Regulatory and coverage policies, in addition to clinical guidelines and recommendations, will determine telehealth use in the future.
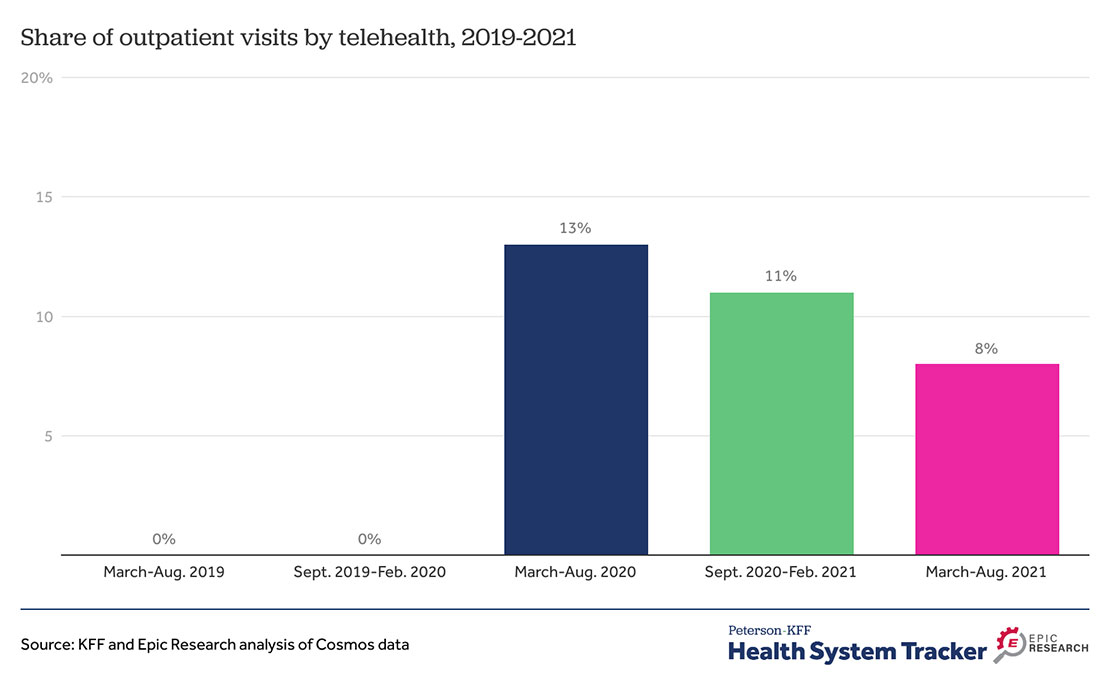
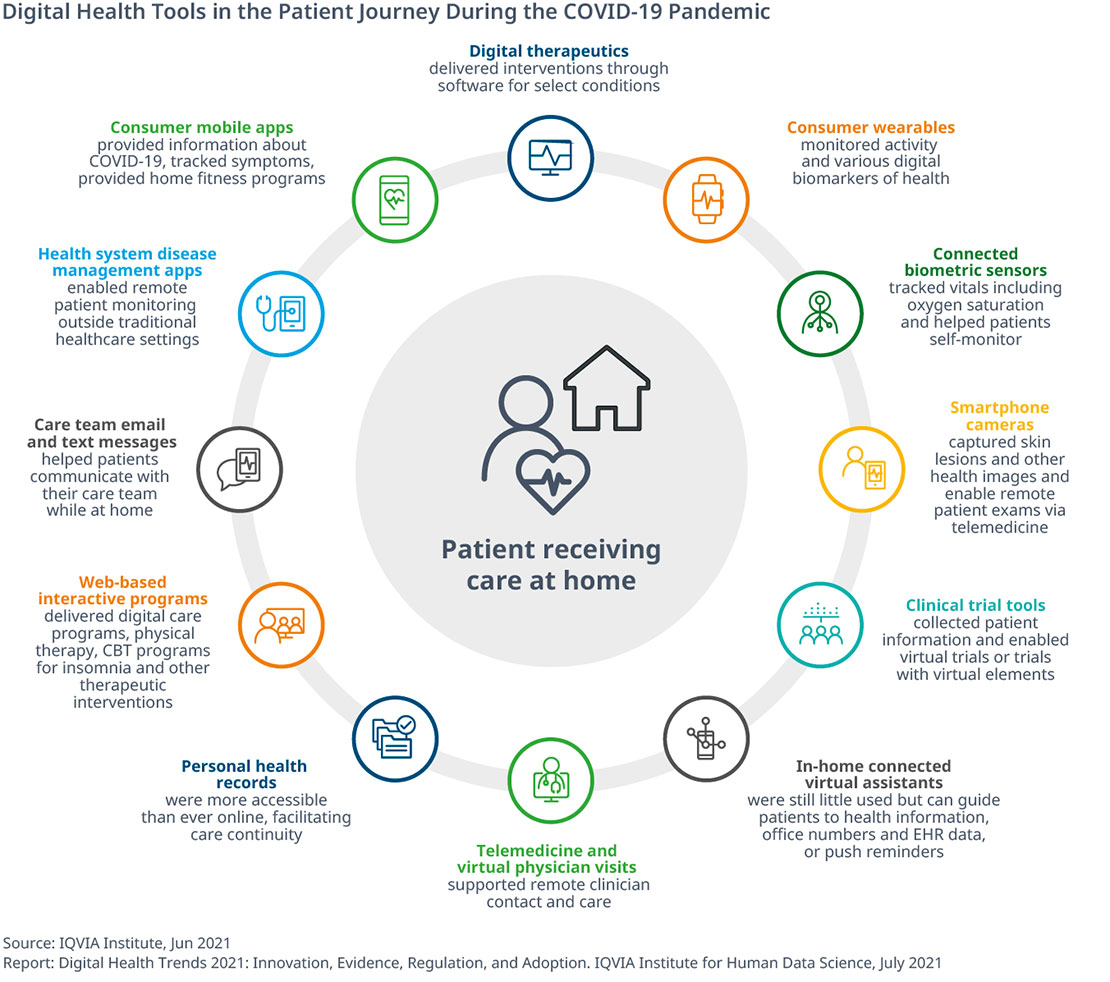
Source: Jen Christensen. ““Federal Government Makes a Push for Community Health Centers to Provide Virtual Health Services Beyond the Pandemic,” CNN Health, 2022.
Source: Justin Lo. ““Outpatient Telehealth Use Soared Early in the COVID-19 Pandemic but Has Since Receded,” Health System Tracker,” Tracker, 2022.
